発表論文
2025
- Doura T., Sugawara T., Inoue H., Tsujikawa M., Michibata Y., Hamachi I., Kiyonaka S. A chemogenetic strategy for inhibiting G protein-coupled receptor activity using metal coordination. Chem. Lett. 54, upaf152 (2025). https://academic.oup.com/chemlett/article/54/8/upaf152/8237815
- 曽我 恭平, 清中 茂樹. ケミカルプローブによるシナプス可塑性時のAMPA受容体動態解析, 医学のあゆみ, 293, 1201-1206 (2025).https://doi.org/10.32118/ayu293131201
- Soga K., Fujiwara T., Nakagawa M., Shibata A., Adriel H., Yatsuzuka K., Kakegawa W., Yuzaki M., Hamachi I., Nango E., Kiyonaka S. Rapid and reversible fluorescent probe enables repeated snapshot imaging of AMPA receptors during synaptic plasticity. Sci. Adv., 11, eadt6683 (2025). DOI: 10.1126/sciadv.adt6683
- Doura T., Sugawara T., Inoue H., Tsujikawa M., Michibata Y., Hamachi I., Kiyonaka S. A chemogenetic strategy for inhibiting G protein-coupled receptor activity using metal coordination. ChemRxiv, 10.26434/chemrxiv-2025-1zq06 (2025).
- 井上 始, 清中 茂樹. 配位化学による細胞膜受容体の自在な制御, 高分子, 74, 25-26 (2025).https://doi.org/10.11477/mf.2425201865
- Ueda Y., Kiyonaka S., Selfors L. M., Inoue K., Harada H., Doura T., Onuma K., Uchiyama M., Kurogi R., Yamada Y., Sun J. H., Sakaguchi R., Tado Y., Omatsu H., Suzuki H., Aoun M., Nakayama T., Kajimoto T., Yano T., Holmdahl R., Hamachi I., Inoue M., Mori Y., Takahashi N. Intratumour oxidative hotspots provide a niche for cancer cell dissemination. Nat. Cell Biol., 27, 530–543 (2025). DOI: 10.1038/s41556-025-01617-w
2024
- Suzuki H., Doura T., Matsuba Y., Matsuoka Y., Araya T., Asada H., Iwata S., Kiyonaka S. Photoresponsive adenosine derivatives for the optical control of adenosine A2A receptor in living cells. ACS Chem. Biol. 19, 2494-2501 (2024) DOI: 10.1021/acschembio.4c00583.
- Soga K., Fujiwara T., Nakagawa M., Shibata A., Adriel H., Yatsuzuka K., Kakegawa W., Yuzaki M., Hamachi I., Nango E., Kiyonaka S. A fluorophore–ligand conjugate for the rapid and reversible staining of native AMPA receptors in living neurons. bioRxiv, 2024.08.21.608930. https://doi.org/10.1101/2024.08.21.608930
- Vaidya R.M., Zhang J., Nall D., Lee Y., Chang Kim E., Ma D., Huang F., Nonaka H., Kiyonaka S., Hamachi I., Jung Chung H., Selvin PR. Nanoscale organization is changed in native, surface AMPARs by mouse brain region and tauopathy. bioRxiv, 2024.07.22.604547. https://doi.org/10.1101/2024.07.22.604547.
- 原 隆史, 堂浦 智裕, 清中 茂樹. 動的構造情報に基づく受容体タンパク質活性制御法の開発, 生体の科学, 75, 269-274 (2024). https://doi.org/10.11477/mf.2425201865
- Doura T., Matsuoka Y., Kiyonaka S. Hijacking endogenous mRNA for genetic code expansion. Nat. Chem. Biol. 20, 660-661 (2024) DOI: 10.1038/s41589-024-01574-9
- Nonaka H., Sakamoto S., Shiraiwa K., Ishikawa M., Tamura T., Okuno K., Kondo T., Kiyonaka S., Susaki E.A., Shimizu C., Ueda H.R., Kakegawa W., Arai I., Yuzaki M., Hamachi I. Bioorthogonal chemical labeling of endogenous neurotransmitter receptors in living mouse brains. Proc. Natl. Acad. Sci. USA, 121, e2313887121 (2024) DOI: 10.1073/pnas.2313887121
- Araya T., Matsuba Y., Suzuki H., Doura T., Nuemket N., Nango E., Yamamoto M., Im D., Asada H., Kiyonaka S., Iwata S.. Crystal Structure Reveals the Binding Mode and Selectivity of a Photoswitchable Ligand for the Adenosine A2A Receptor. Biochem. Biophys. Res. Commun. 695, 149393 (2024) DOI: 10.1016/j.bbrc.2023.149393
2023
- Araya T., Matsuba Y., Suzuki H., Doura T., Nuemket N., Nango E., Yamamoto M., Im D., Asada H., Kiyonaka S., Iwata S.. Crystal Structure Reveals the Binding Mode and Selectivity of a Photoswitchable Ligand for the Adenosine A2A Receptor. ChemRxiv, 10.26434/chemrxiv-2023-7q2ht (2023).
- Suzuki H., Doura T., Matsuba Y., Matsuoka Y., Araya T., Asada H., Iwata S., Kiyonaka S. Photoresponsive adenosine derivatives for optical control of adenosine A2A receptor in living cells. ChemRxiv, 10.26434/chemrxiv-2023-z5pnd (2023).
- 清中 茂樹, 堂浦 智裕, 高次脳機能の理解に向けた分子標的ケモジェネティクス法の開発, MEDCHEM NEWS, 33, 136-141 (2023). DOI: 10.14894/medchem.33.3_136
- 清中 茂樹, 鈴木 啓文, 細胞種選択的なグルタミン酸受容体の活性化手法「配位ケモジェネティクス」の開発, 生物物理, 63, 157-159 (2023). DOI:10.2142/biophys.63.157
- 松岡 佑真, 柏 俊太朗, 堂浦 智裕, 清中 茂樹, 高次脳機能の理解に向けた次世代ケモジェネティクス法の開発, Brain and NERVE, 75, 367-374 (2023). DOI:10.11477/mf.1416202342
- Nonaka H., Mino T., Sakamoto S., Oh J.H., Watanabe Y., Ishikawa M., Tsushima A., Amaike K., Kiyonaka S., Tamura T., Aricescu A.R., Kakegawa W., Miura E., Yuzaki M., Hamachi I. Revisiting PFA-mediated tissue fixation chemistry: FixEL enables trapping of small molecules in the brain to visualize their distribution changes., Chem, 9, 523–540 (2023). DOI:10.1016/j.chempr.2022.11.005
- 三浦 裕太, 清中 茂樹, 化学で脳を理解するための新技術「配位ケモジェネティクス」, 化学, 78, 24-29 (2023)
2022
- 三浦 裕太, 小島 憲人, 清中 茂樹, 配位ケモジェネティクスによるグルタミン酸受容体の活性制御, 日薬理誌, 157, 366-370 (2022)
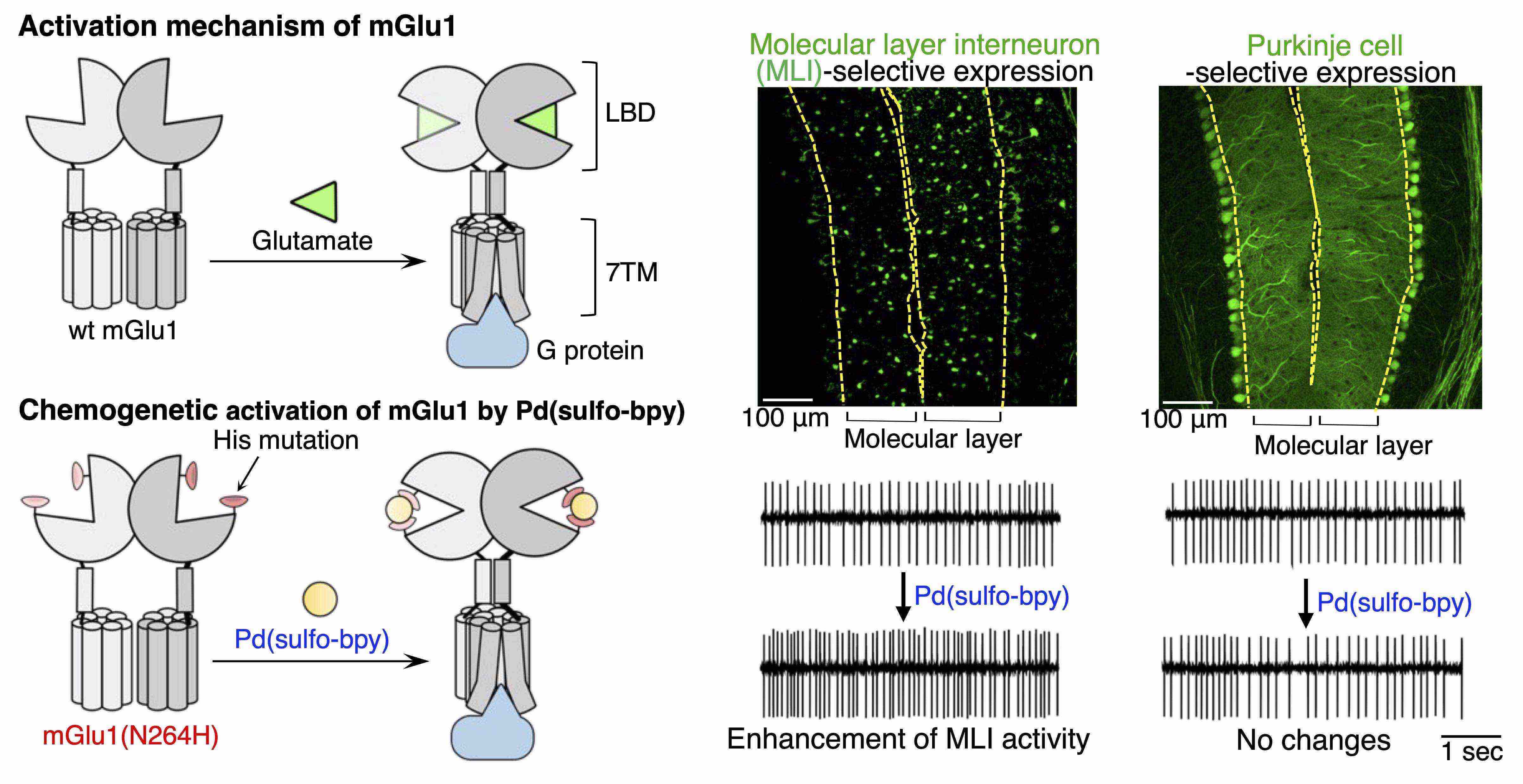
- Ojima K., Kakegawa W., Yamasaki T., Miura Y., Itoh M., Michibata Y., Kubota R., Doura T., Miura E., Nonaka H., Mizuno S., Takahashi S., Yuzaki M., Hamachi I., Kiyonaka S. Coordination chemogenetics for activation of GPCR-type glutamate receptors in brain tissue. Nat. Commun., 13, 3167 (2022). DOI: 10.1038/s41467-022-30828-0
- 杉原 佑太朗, 小島 憲人, 清中 茂樹, リガンド指向性2段階ラベル化によるAMPA型グルタミン酸受容体の精密動態解析, 日薬理誌, 157, 191-195 (2022)
- 曽我 恭平, 清中 茂樹, 迅速ケミカルラベル化法によるAMPA型グルタミン酸受容体の精密動態解析, 生化学, 94, 292-297 (2022)
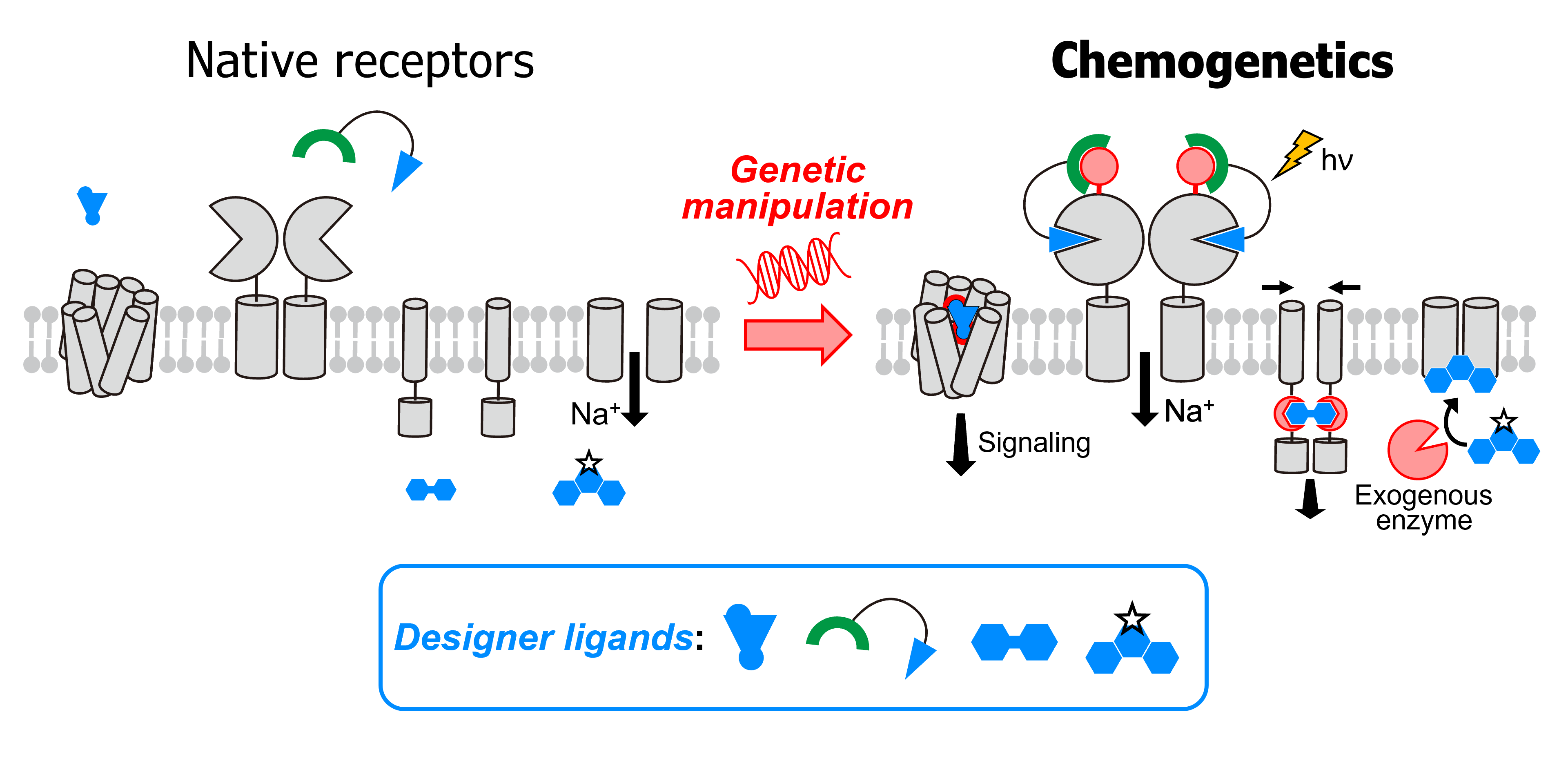
- Miura Y., Senoo A., Doura T., Kiyonaka S. Chemogenetics of cell surface receptors: beyond genetic and pharmacological approaches. RSC Chem. Biol., 3, 269-287 (2022). DOI: 10.1039/d1cb00195g.
- 妹尾 暁暢, 清中 茂樹, 配位ケモジェネティクスによる代謝型グルタミン酸受容体の制御, 化学と工業, 75, 115 (2022)
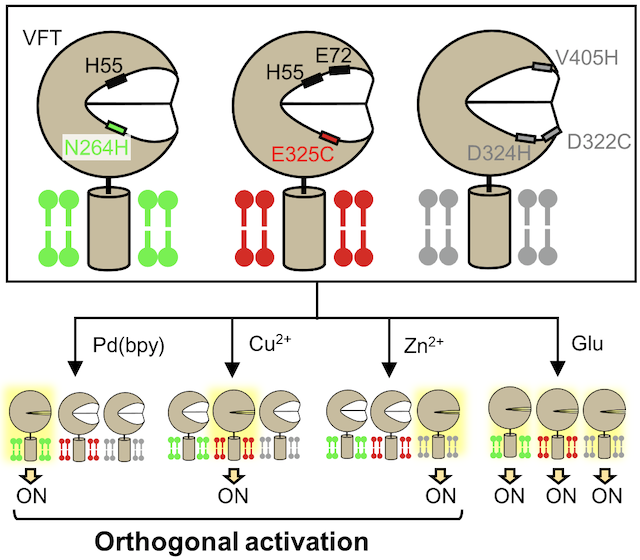
- Senoo A., Yamada Y., Ojima K., Doura T., Hamachi I., Kiyonaka S. Orthogonal activation of metabotropic glutamate receptor using coordination chemogenetics. Front. Chem., 9, 825669 (2022). DOI: 10.3389/fchem.2021.825669.
2021
- 坂口 怜子, 金岡 英徳, 清中 茂樹. タンパク質温度センサーを利用した細胞内温度計測 熱測定, 48, 152-158 (2021)
- Tsai Y.H., Doura T., Kiyonaka S. Tethering-based chemogenetic approaches for the modulation of protein function in live cells. Chem. Soc. Rev., 50, 7909-7923 (2021). DOI: 10.1039/d1cs00059d.

- 三浦 裕太, 清中 茂樹, GPCRおよびGタンパク質シグナルを特異的に制御するケモジェネティクス法, 細胞, 53, 328-330 (2021).
- Ojima K., Shiraiwa K., Soga K., Doura T., Takato M., Komatsu K., Yuzaki M., Hamachi I., Kiyonaka S. Ligand-directed two-step labeling to quantify neuronal glutamate receptor trafficking. Nat. Commun., 12, 831 (2021). DOI: 10.1038/s41467-021-21082-x.

- Kojima Y., Okuzaki Y., Nishijima K., Moriwaki S., Asai S., Kaneoka H., Iijima S. Regulatory mechanism of chicken lysozyme gene expression in oviducts examined using transgenic technology. J. Biosci. Bioeng., 131, 453-459 (2021). DOI: 10.1016/j.jbiosc.2020.11.011
2020
- Hagihara Y., Okuzaki Y., Matsubayashi K., Kaneoka H., Suzuki T., Iijima S., Nishijima K. Primordial germ cell-specific expression of eGFP in transgenic chickens. Genesis., 58, e23388 (2020).
- Aoyama H., Doura T. Selective acetylcholinesterase inhibitors derived from muscle relaxant dantrolene. Bioorg. Med. Chem. Lett., 30, 126888 (2020). DOI: 10.1016/j.bmcl.2019.126888.
2019
- Tateyama H., Murase Y., Higuchi H., Inasaka Y., Kaneoka H., Iijima S., Nishijima K. Siglec-F is induced by granulocyte–macrophage colony-stimulating factor and enhances interleukin-4-induced expression of arginase-1 in mouse macrophages. Immunology, 158, 340-352 (2019). DOI: 10.1111/imm.13121
- Sakamoto S., Yamaura K., Numata T., Harada F., Amaike K., Inoue R., Kiyonaka S., Hamachi I. Construction of a Fluorescent Screening System of Allosteric Modulators for the GABAA Receptor Using a Turn-On Probe. ACS Cent. Sci., 5, 1541-1553 (2019). DOI: 10.1021/acscentsci.9b00539.
- Okuzaki Y., Kaneoka H., Suzuki T., Hagihara Y., Nakayama Y., Murakami S., Murase Y., Kuroiwa A., Iijima S., Nishijima K. PRDM14 and BLIMP1 control the development of chicken primordial germ cells. Dev. Biol., 455, 32-41 (2019). DOI: 10.1016/j.ydbio.2019.06.018
- Doura T., Nishio T., Tamanoi F., Nakamura M. Relationship between the glutathione-responsive degradability of thiol-organosilica nanoparticles and the chemical structures. J. Mater. Res., 34, 1266-1278 (2019). DOI:org/10.1557/jmr.2018.501
- Kubota R., Kiyonaka S., Hamachi I. On-cell coordination chemistry: Chemogenetic activation of membrane-bound glutamate receptors in living cells. Methods Enzymol., 622, 411-430 (2019). DOI: 10.1016/bs.mie.2019.02.033.
- Sakamoto S, Kiyonaka S, Hamachi I. Construction of ligand assay systems by protein-based semisynthetic biosensors. Curr. Opin. Chem. Biol., 50, 10-18 (2019). DOI: 10.1016/j.cbpa.2019.02.011.
- Mekaru H., Yoshigoe A., Nakamura M., Doura T., Tamanoi F. Biodegradability of disulfide-organosilica nanoparticles evaluated by soft X-ray photoelectron spectroscopy: cancer therapy implications. ACS Appl. Nano Mater. 2, 479-488 (2019). DOI: 10.1021/acsanm.8b02023